Your Nose is the entry gate for viruses & contaminants. Use of VirX NONSTM delivers Nitric Oxide to the Nasal cavity which creates a hostile microenvironment in the nose where airborne viruses can’t survive. VirX allows for intranasal delivery of a topical, non-systemic, small amount of Nitric Oxide that is lethal to microbes but safe to humans.

Physically it forms a protective layer and chemically low pH inactivates the viruses
The compounds used in the liquid are all used extensively in the food.
It reduces viral load 16 times higher than saline nasal spray and ensures a lasting protection.
Nitric Oxide in VirX Nasal are same as the body forms, also it is found primarily in green leafy vegetables.
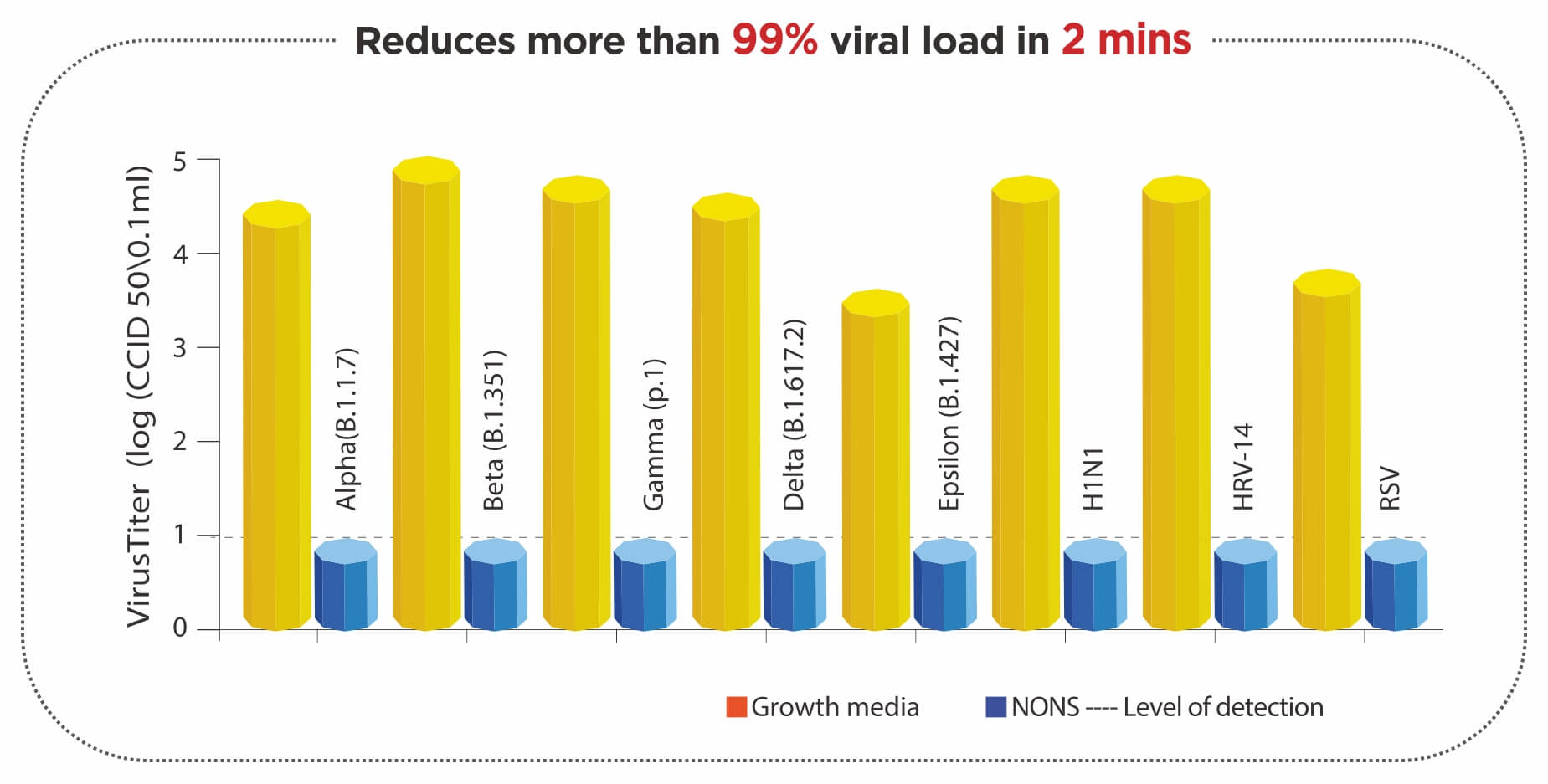
Reference: Based on the result of Indian Phase 3 Clinical Trial - Data on file and standard supportive care was in accordance with latest guidelines issued by Ministry of Health and Family Welfare; Government of India.
Reference: Indian Phase 3 Clinical Trial- Standard supportive care was in accordance with latest guidelines issued by Ministry of Health and Family Welfare; Government of India.
